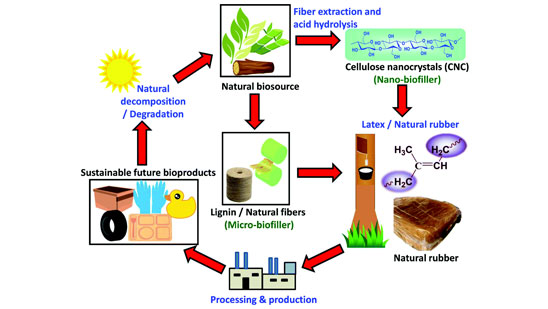
Carbon blacks, the most widely used class of reinforcing fillers in rubber industry, are manufactured from finite fossil fuel sources. Unsustainable consumption of fossil fuel is an environmental concern and automotive industries are therefore reducing fuel usage in order reduce carbon emissions. As the world moves away from the utilization of fossil fuels for mobility, rubber industries are also evaluating fillers from sustainable resources in an attempt to reduce their dependency on carbon blacks. Efforts are on to increasingly use sustainable fillers such as cellulose and precipitated silica in place of carbon black as these natural materials are available in abundance. There are, however, fundamental challenges in using these materials in rubber compounds. Cellulose need to be significantly modified in terms of its particle size and surface chemistry. Size of cellulose particles are far higher than required to be considered as reinforcing filler for elastomers. Physico-chemical nature of cellulose is more akin to that of precipitated silica in that chains in the former are extensively hydrogen bonded, highly self associating, crystalline in nature and therefore very difficult to disperse them in elastomers in a compatible manner to realize their reinforcing potential. Hydroxyl groups are not easily accessible for suitable surface modifications. Similarly, the strong self-associating nature of silica leads to the formation of network structures among themselves resulting in poor rubber-filler interactions, poor dispersion, and high compound viscosity all posing serious processing difficulties. Silanization with coupling agents improves silica-elastomer interactions, decreases filler-filler interactions, and reduces compound viscosity and enable easier processing with better vulcanizate properties. Mixing and silanization of precipitated silica is an energy intensive process and realizing quality dispersion of silica in rubbers in an energy efficient manner remains a challenging task. At higher silica loading, significant increase in viscosity makes flow of such compounds more difficult and causes wear on the processing equipment. It is a fact that the relatively high temperature window for mixing silica compounds is quite narrow as silanization decreases at low temperatures while the risk of scorch is real at higher temperatures. Though silane coupling agents have significantly helped in realizing the reinforcing potential of precipitated silica, challenges increased Mooney viscosity, scorch, dispersion, mixing energy, polymer degradation, and ethanol emissions remain to be addressed in a sustainable manner. such
Recent efforts on the modification of both cellulose and silica using ionic liquids are discussed in this presentation to understand mixing of these materials with elastomers.